Wong Lab Develops Smarter Cancer-Killing Cells
A system of engineered cells that communicate, targeting autoimmune diseases too
By Patrick L. Kennedy
New research out of the College of Engineering could enable doctors to program a patient’s immune system with a more versatile toolkit than ever before in the fight against cancer, as well as autoimmune diseases. Associate Professor Wilson Wong (BME) and colleagues published their study this month in Nature Communications.
Wong’s team approaches the immune system as a computing circuit, which in many ways it resembles. They are reprogramming the circuit to identify and eliminate tumor cells that it’s not catching automatically. Existing immunotherapies do this, but not with the same simultaneous level of specificity and broadness as Wong’s system.
This latest development in synthetic biology is part of Wong’s ongoing work to improve upon chimeric antigen receptor (CAR) T-cell treatments. In conventional CAR-T therapy, T-cells are extracted from a patient’s blood sample and then modified to attack specific antigens (receptor molecules) on the patient’s tumor cells. But the treatment can backfire by triggering a massive release of cell-killing substances called cytokines, which can be life-threatening.
What Wong’s lab has developed in recent years is a CAR-T system that is split, universal, and programmable (SUPRA). That means it can target two types of antigens, making it effective against a wider variety of cancers, and it can be deactivated to prevent cytokine-induced side effects.
In their new study, Wong’s team further tweaked their CAR-T cells by adding a third target antigen, meaning the engineered immune cells can operate with a higher degree of specificity. To explain the advantages of being more specific, Wong poses the analogy of a detective searching for a crime suspect. If all he has to go on is that the suspect “wears glasses,” that doesn’t narrow the field much. If he learns he’s looking for someone with glasses and a beard, he might be getting somewhere. Now, if the suspect has glasses, a beard, and a purple beret, then the detective has a clear idea of what he’s looking for.
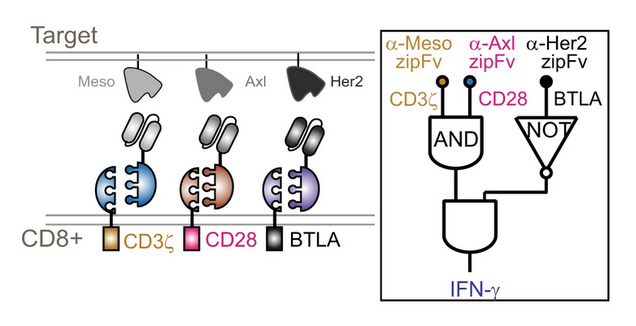
Wong’s cancer-killing T-cells, similarly, are more effective because they know what characteristics (in their case, antigens) they’re really looking for. They can even be programmed with what the team calls “NOT logic,” ordering them to search for “A and B and not C.” (So glasses and beard, but not the purple beret.)
Moreover, Wong’s team showed that their SUPRA system works not just in T-cells but in multiple immune cell types. That should pave the way for safer therapies for cancer as well as autoimmune disorders such as arthritis and multiple sclerosis. The system can also suppress the immune response that leads to organ transplant rejection.
It’s worth noting that Wong’s lab accomplished these advances by applying distributed computing principles to the engineered immune cells as a system. “In computer science, distributed computing is about having multiple computers that all do pieces of the computation, and then they combine to solve the bigger problem—that way, each computer doesn’t need to be a super-computer,” Wong explains. “We’ve kind of done that by splitting the parts of the computation among different cell types and having them come together to form a program.”
Finally, the cells are directed to target specific antigens by way of a new synthetic intracellular communication channel that Wong and colleagues have engineered. “Each cell type is tasked with sensing and producing a specific subset of inputs and outputs,” the co-authors write in the paper. “Immune cells can directly communicate with each other to attain temporally choreographed responses.”
Wong’s collaborators on the study were James J. Collins, the former ENG professor who pioneered synthetic biology; former PhD student Jang Hwan Cho (’19); former ENG postdoc Atsushi Okuma; former master’s student Katri Sofjan (’19); and PhD student Seunghee Lee. The SUPRA technology is licensed to Senti Bioscience, co-founded by Wong and Collins.
The full article is available at the Nature Communications website.
