Our Recent Work
Recent publication
Student researchers in the Partnership for Global Health Technologies have published an article on their work modeling how oxytocin availability is related maternal health outcomes:

Nadkarni et al. Modeling patient access to therapeutic oxytocin in Zanzibar, Tanzania (2018)
Summer 2017
In July 2017, Partnership for Global Health Technologies student researchers made their third annual trip to Tanzania to continue work on our projects focused on quality control of malaria rapid diagnostics and dynamic systems modeling of health facilities.
This year, we continued our collaboration with State University of Zanzibar medical students and Mnazi Mmoja Hospital clinical staff in Stone Town, Zanzibar. Additionally, we expanded the scope of our research, conducting research on mainland Tanzania in collaboration with Muhimbili University of Health and Allied Sciences.
Quality control of malaria rapid diagnostics
The quality control of malaria rapid diagnostic tests (mRDT) project aims to assess the quality of mRDT by utilizing image processing techniques to distinguish between high-quality and defective diagnostics from images taken of the tests. The goal is to develop an algorithm that can be used as a non-destructive method for assessing the quality of mRDT at the point-of-care, while also providing a quantitative metric for reading mRDT results that eliminates potential user bias.

This summer four undergraduate students from Boston – three from Boston University and one from Harvard University – and a SUZA second year medical student traveled to Bagamoyo, Tanzania to collect data on the quality of malaria rapid diagnostic tests and the validity of their results. The student researchers hypothesized that there are visual differences between valid and invalid mRDT that can be detected using image analysis. Therefore, an imaging device was designed and 3D printed to be used on-site at Bagomoyo District Hospital to collect up-close images of the mRDT before, during, and after the malaria tests were run. The imaging device was controlled using a Raspberry Pi running Python script, which took images at predetermined intervals.
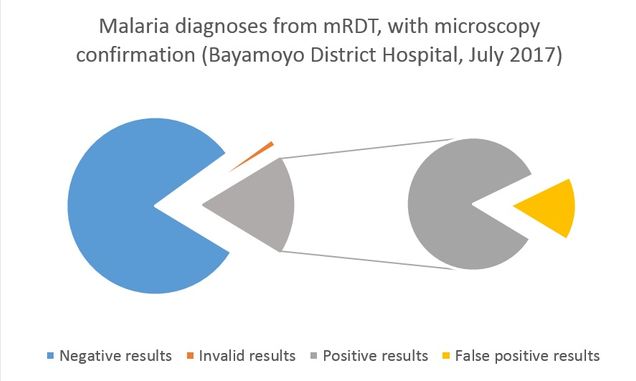
Over the span of 21 days, images of 210 mRDT were collected, with 60 images collected per test. For each mRDT that was imaged, a blood sample was collected from the patient to conduct confirmatory diagnoses using microscopy and PCR. Microscopists in Tanzania – at Bayamoyo District Hospital and Muhimbili University – provided the first level of confirmatory diagnosis on site. Blood spots from the participating patients were shipped to Boston University where PCR analysis will be conducted. From the mRDT data collected, there were a total of 179 negative diagnoses, 32 positive diagnoses, and 3 invalid result. When compared with the first microscopy confirmatory results, there were 6 false positive results, where the microscopist found no malaria present in the sample that tested positive on mRDT. We will re-evaluate our results once the PCR analysis and second microscopy reading are complete.
Next steps
During the Fall 2017 semester, student researchers will analyze the dried blood spots using PCR and compare those results to the microscopy and mRDT diagnoses to identify possible mismatches. This will provide a better sense of how the CareStart Malaria HRP-2/pLDH (Pf/pan) Combo Test performed in relation to the gold standard laboratory diagnostic techniques – PCR and microscopy. Following this, a clustering analysis will be performed on the images collected to see which one is most successfully able to distinguish and group together images that might be correlated with an invalid mRDT diagnosis.

In parallel with the data analysis, a modified and improved version of the imaging box used in summer 2017 will be designed and 3D printed. This imaging box will serve a dual purpose. First, additional mRDT samples will be tested after these mRDT have been exposed to different conditions – such as heat, humidity and pressure – in order to gather more information about how these factors affect the mRDT. Second, the imaging box will be more adaptable to imaging mRDT of different brands; the imaging box used over this past summer was specifically designed for the CareStart mRDT. This will allow for additional testing across multiple brands of mRDT.
Dynamic modeling of health systems at the facility level

This summer, researchers from the PGHT team collected data to validate their model, simulating operations in a maternity ward while taking into account patient condition upon intake, changes in patient condition due to treatment provided, and the human and material resources available for treatment. A model with this level of specificity and comprehensiveness will allow hospital administration to explore how certain adjustments to resource allocation will impact maternal mortality, and therefore identify areas where funds will be most effective in lowering the number of casualties in the maternity ward.
The team first met with collaborators, Drs. Tarek Meguid and Tanneke Herklots, to determine the best method of data collection to inform the model. Since the goal of the model is to utilize information about the maternity ward to optimize resource allocation levels for reduced maternal mortality, researchers sought to collect data on the patients who required the most resources – the ‘potentially life threatening cases’ (PLTC) and the ‘maternal near misses’ (MNM). As part of her work studying MNMs, Dr. Herklots has been collecting PLTC patient records as hard copy patient files. These files also contained the information we needed, but because it was not feasible to extract each individual data point from the approximately 800 files during our six-week stay in Stonetown, we decided that scanning the files and uploading them to a shared drive would be the best way to access the information later, for both Herklots’s study and for our modeling purposes.

Using a smartphone compatible app, researchers were able to obtain clear scans of the files in an efficient manner and upload them to the shared drive. The process of scanning involved first reviewing the file to determine whether it was a MNM or just a PLTC, and ensure that it was within the desired date range of July 2016-December 2016. Then the files were scanned and stored by date and by condition, (i.e. pre-eclampsia, anemia, or postpartum hemorrhage). Once researchers had scanned a majority of files, they began constructing a database to convert data into a format compatible with the model code.
To get a more complete picture of the functionality of the maternity ward, researchers collected additional information about cases that did not fall into the PLTC category. To do this, researchers reviewed partogram files, along with the corresponding admission forms and delivery records, for each pregnant patient who delivered at the hospital over a one-month time period. Data was also collected on ward operations, such as the numbers and position of staff throughout the day, the number of admitted patients, and the number of patients discharged to determine ratios of staff to patients.
To supplement the raw data obtained from patient files, researchers attended daily hospital staff meetings to better understand the context of what they were attempting to model. They were also able to interview hospital staff – from nurses to physicians to pharmacists – who provided valuable information about facility capacity, gaps in resources, and planned operational changes. Finally, researchers – led by the State University of Zanzibar medical students – spoke directly with maternity patients to learn more about their experience in the hospital’s maternity ward.
The data collected – with generous help from the staff at Mnazi Mmoja Hospital – will not only supply the necessary data for the model but will also inform what the model examines; review of patient records led researchers to alter the original list of included conditions and to better understand common co-conditions and their impact on patients and their treatment regimens in the ward. The information collected is critical to producing accurate and relevant output from the model, ensuring it can be used as a decision-making tool in the context of maternity wards in low-income countries.
Summer 2016
Students participating in the field portion of the Partnership for Global Health Technologies program spent six weeks working closely with State University of Zanzibar medical students and local Zanzibari healthcare professionals on projects that addressed public health challenges identified the previous summer.

Designing a paper-based test for alanine aminotransferase (ALT)
Preeclampsia affects up to 10% of pregnant women worldwide, and has the potential to cause morbidity and death in both the mother and the child. While preeclampsia is common in high, middle and low income countries, it’s countries with limited resources that have difficulty managing preclampsia before, during, and sometimes after delivery. In addition to crumbling health infrastructure, poor supply chain management and inadequate healthcare workforce, management of preeclampsia is further complicated by the stage at which many women in poor countries access healthcare services. Due to a number of factors, including inability to pay for services, lack of transportation to health facilities and lack of knowledge on the condition and its symptoms, women often delay treatment and present with severe symptoms of preclampsia, and sometimes eclampsia. Undiagnosed and unmanaged preeclampsia can lead to renal and liver function complications in the mother.

This study aims to improve monitoring of liver function in preeclamptic women by evaluating the adoptability of our point-of-care device for monitoring alanine aminotransferase (ALT), an important liver biomarker.
In order to evaluate the adoptability and user interaction of our device, a non-functioning look-alike prototype was made, along with a brochure explaining how the device will be used. Use of the device was demonstrated before students administered a survey crafted to collect information from:
- Physicians on the standard method of liver function screening, liver-related medical device distributions, and costs of screening.
- Laboratory technicians on the protocol of monitoring liver problems and cost of monitoring.
- Patients on care seeking behaviors, ability to afford care, reliability of the type of care, and the problems they encounter in receiving care
Students will spend the upcoming semester analyzing survey results and incorporating their findings into the design of a fully functioning prototype that will be tested in Zanzibar summer 2017.
Investigating the Quality of Rapid Diagnostic Tests (RDT) for Malaria

There is concern over the quality assurance of malaria rapid diagnostic tests, and this issue has led to mistrust of the technology as well as misinterpretation of test results. As a result, various methods have been proposed and are currently being explored that may improve quality assurance for all brands of rapid diagnostic tests. In this study, we are aiming to assess quality control methods used in real time situations and to explore a new methods for quality assurance in the field. We plan to take photos of rapid diagnostic tests used for malaria, before and after their use, and will use image processing techniques to determine if there are quantifiable characteristics between well manufactured rapid diagnostic tests and defective rapid diagnostic tests. We will compare those results provided by rapid diagnostic tests to the microscopy confirmation results to determine rapid test quality, while ensuring patient information collected is unidentifiable.
BU students visited a number of health facilities where patients were tested for malaria using a RDT. Before each patient was tested for malaria an image of the RDT was taken using a 3D printed imaging container and a cell phone with a microscope lens attachment. The start time of the blood and buffer added to each RDT cassette was recorded onto the data collection sheet. Once the test was administered and the results recorded by the healthcare professional, a second image of the RDT was taken using the imaging container. Students will work on analyzing these images in the upcoming semester.
Summer 2016 Student Reports
