Ultrasound-Mediated Blood-Brain Barrier Opening
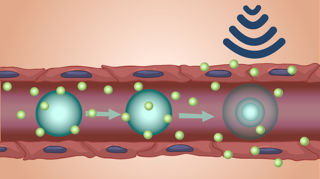
Forced oscillations driven by ultrasound can be used for transient and focal opening of the BBB. First reported more than two decades ago, this approach has been utilized for delivery of free and encapsulated drugs and biologics, nanoparticles, and a few cell types. There are multiple phase 0/1 clinical trials underway to test the safety and repeatability of the technique in humans as the first step towards testing the efficacy of ultrasound-mediated drug delivery for treatment various brain diseases, including cancer and Alzheimer’s disease. Researchers within the NanoMedAL plan to study the influx and clearance rates for circulating drugs after opening the BBB. We also intend to quantify the percentage of administered therapeutic that crosses the disrupted BBB and assess its spatial distribution as a function of physicochemical properties of the therapeutic (i.e. nanoparticle size and surface charge or solute molecular weight). The findings from these studies can guide the therapeutic dose administered as well as the frequency of BBB opening in order to optimize the therapeutic effect.