Expanding the Scope of Research | New Nikon CSU-W1 SoRa Microscope to advance research potential across BU
By: Danny Giancioppo | Photo Credit: Kelly Peña
If you go to the second floor of the Boston University Life Science and Engineering Building, you are apt to find researchers working in a dark-curtained room in the biology wing dedicated to the newly acquired Nikon CSU-W1 SoRa microscope (to learn more about accessing the microscope, see the dedicated College of Engineering page). This $660k piece of tech, acquired with help from the Boston University Photonics Center, is a considerable upgrade to most microscopes in the field. Using a high-speed spinning disk, background lighting from different depths is avoided by utilizing a series of pinholes around the disks to neatly capture images of thin planar sections of 3D biological specimens stained with different fluorescent probes. With the super resolution disk option, the images are accessible at a much higher quality than ever before––an image that is 1.4 times greater spatial resolution than the optical limit.
The need for such a microscope came to be over a year ago, when Photonics director Dr. Thomas Bifano reached out to faculty inquiring about the need for a mesoscope out of Thorlabs, Inc., which uses a gantry to orbit around an object, providing multiple angles without the need to disturb the object itself. However, professors Jerry Chen (Biology), Jeroen Eyckmans (Biomedical Engineering), and Jennifer Luebke (Anatomy & Neurobiology) came back to Dr. Bifano expressing the need for a piece of hardware more like Nikon’s CSU-W1 SoRa. Such a microscope could advance the study of transcriptomics (the study of transcriptomes; the sum of messenger RNA molecules expressed from the genes of an organism) invaluably, as well as further develop the fields of tissue engineering and connectomics (the study of the brain’s structural and functional connections and communication between groups of neurons).
“Spatial biology is an exploding area of research in which we are trying to understand molecules such as DNA, RNA, or proteins in the three-dimensional context of the tissue in which they exist,” explains Professor Chen. “You need to track molecules that are less than a micron in size across cells and tissues of organs that are several cubic millimeters or centimeters in volume.” In Chen’s view, the Nikon CSU-W1 SoRA microscope “has both the resolution and imaging speed to capture images that span these spatial scales in a timely manner that is practical for researchers at BU for their everyday experiments.”
As a result, the Photonics Center’s objective was simple: get the faculty their microscope.
“The core value of the Photonics Center is that we offer lots of shared equipment that people can use without having to pay for the capital expense of getting it here,” Dr. Bifano explains, adding that BU does not issue user fees as a gateway to accessing their units, unlike centers at some other universities.

Through this focus, the Photonics Center was able to receive the microscope for 70% of its total unit and tech support costs, paying for the remaining 30% on their own. Thereafter, the microscope was decidedly placed on the second floor of the LSEB (room 222) under the aid and instruction of Biology Chair Pam Templer, as it would be best located there for its frequent users. Now it is being overseen by Dr. Zahid Yaqoob, who has recently joined the Boston University Biomedical Engineering (BME) department as Research Associate Professor. Before joining the BME department, Dr. Yaqoob was at the MIT Laser Biomedical Research Center (LBRC) where he led a team of researchers developing advanced interferometric optical imaging tools for pathophysiological studies in single cells and biological tissue samples.
 As Director of Micro/Nano Imagining (MNI) Facility (BME) and Technical Director of the Neurophotonics Center, Dr. Yaqoob is involved in the design and development of optical imaging platforms for neuroscience applications and beyond, as well as training researchers at an undergraduate, graduate, and postdoctoral level on the proper usage of instruments such as Nikon CSU-W1 SoRa microscope.
As Director of Micro/Nano Imagining (MNI) Facility (BME) and Technical Director of the Neurophotonics Center, Dr. Yaqoob is involved in the design and development of optical imaging platforms for neuroscience applications and beyond, as well as training researchers at an undergraduate, graduate, and postdoctoral level on the proper usage of instruments such as Nikon CSU-W1 SoRa microscope.
Following proper training from Dr. Yaqoob, researchers will gain key card access to the room where the microscope has been set up alongside blackout curtains and adjustable lighting fixtures. In accordance with the pre-existing scheduling system for MNI facility, users will be able to reserve the microscope as an imaging resource, when available, in the appropriate manner. Suffice to say, this microscope is one which students will not want to miss the chance of incorporating into their research.

“The microscope is ideal for super-resolution (~1.4x the optical limit) fluorescence imaging of biological samples at high-speed (up to 200 full frames/secs),” Yaqoob says. The high frame rate is possible due to the spinning disk (with multiple confocal pinholes) feature of the CSU-W1, as compared to a laser scanning confocal microscope that can capture only one pixel at a time.
Of course, spinning disks are a common feature of many modern microscopes. However, the CSU-W1 has a new pinhole design, which “optimizes the pinhole size and spacing to significantly reduce the pinhole crosstalk,” Yaqoob explains, “which is particularly important when imaging thicker samples with multiple scattering.” The CSU-W1 also features a field-of-view nearly four times wider than average for large area scanning applications.
Professor Jeroen Eyckmans has praised some of these capabilities, and how they might advance the understanding of cellular creation of vascularized tissue. “The SoRa has the capacity and resolution to capture real-time images of living cells as they assemble fibrillar extracellular matrix and develop vascular networks. By using fluorescent-based molecular sensors that report fiber tension, cell-cell junction formation and shear stress, we will be able to map for the first time how mechanical forces at the molecular level control the creation and function of vascular tissue.”
Additionally, the CSU-W1 comes with a SoRa disk, which replaces the emission pinhole disk with its additional lenslet array, effectively doubling the cone angle on each pinhole, therefore reducing the spot size on an image and increasing resolution. The Nikon CSU-W1 microscope has the additional feature of 2.8x magnification with a 100x objective lens or 4x magnification with a 60x objective lens, allowing for microscopic oversampling. As Dr. Yaqoob explained, “this is particularly beneficial for deconvolution, a computational image processing technique, to further improve the resolution and contrast of images acquired using the Nikon CSU-W1 SoRa microscope.” By using both magnification and microlensing, a “1.4x improvement beyond the optical limit can be achieved,” says Yaqoob. And these are not the only options for magnification without cost to resolution.
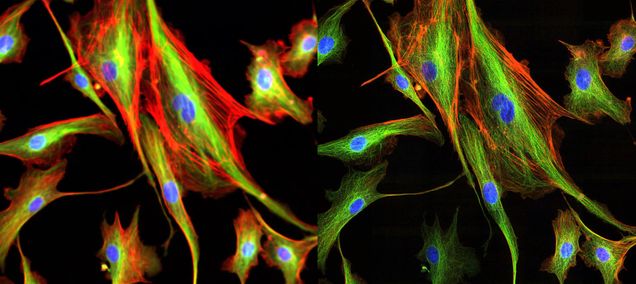
The use of deconvolution, the process by which blur and other visual noise is removed or reduced, can be used to further increase the spatial resolution of the microscope. The CSU-W1 SoRa microscope comes with a variety of deconvolution algorithms, including Richardson-Lucy, Maximum Likelihood Estimation, and Wiener filtering. Each algorithm uses an estimated point spread function (PSF) to reverse the blurred effect of raw images. Furthermore, a built-in artificial intelligence-based algorithm is available to remove noise from raw images. When these features are combined with the SoRa technology’s 1.4x resolution improvement, the CSU-W1 SoRa can reach up to 2x spatial resolution beyond the optical limit.
“The microscope can be operated in both single camera and dual camera modes,” Yaqoob says on the functionality of the CSU-W1. “In single camera mode, multiple fluorophores (channels) are acquired one at a time. The dual camera mode can allow imaging two channels simultaneously, provided the fluorophores are appropriately selected. Any applications where the two fluorescence channels can be effectively separated and directed to two different cameras will essentially improve the imaging speed by 2x.”
Although the microscope has only been in use for the past couple months, the quality of imaging it produces is undeniably lucrative for trained students and faculty alike. It is anticipated to have a variety of integral uses for researchers across the branching fields within Boston University’s BME department, such as Anatomy & Neurobiology, as explained by Dr. Jennifer Luebke.
“We perform immunolabeling of a variety of synaptic markers on fluorescently labeled neurons to assess age-related changes to synaptic markers and receptors across morphological compartments,” says Luebke. “To date we have been employing up to three channel conventional confocal laser scanning imaging methods to assess the filled neurons and surrounding neuropil. At confocal resolution, we can only guess whether punctal appositions are indeed synapses or appositions. Use of by the Nikon CSU-W1 SoRa super-resolution microscope system will enable us to determine the precise localization of many diverse markers associated with neurons and within the neuropil of large areas of the primate neocortex for the first time, thus markedly enhancing the precision and scope of our studies.”

“When you have something super advanced that you can put in a shared facility,” Dr. Bifano says, “that’s pretty powerful at a university.” In some cases, the effort to procure shared equipment can feel imbalanced with the frequent (or infrequent) use of the technology. In the case of the CSU-W1 SoRa microscope, however, Dr. Bifano believes there will be no such concern. “I have a feeling that this is going to be one that is in high-demand and frequently used,” Bifano says. “We’ll wear it out before it dies.”
