Functional Ultrasound Imaging
Innovations in ultrasound technology always has enormous potential. Today, thanks to the technological breakthrough of massive parallel data acquisition and computing, ultrafast ultrasound imaging has been changing the paradigm in vasculature imaging[1]–[3]. In December 2017, the BU Neurophotonics Center acquired an ultrafast ultrasound imaging system (Vantage 256, Verasonics Inc.). We have implemented several novel imaging techniques and been applying these technologies for brain functional and pathologic studies.
Ultrafast ultrasound coherent plane wave compounding
A conventional ultrasound image is usually acquired by sequential scanning a focused beam across the image plane and a 2D image is typically made of 64-512 such lines. Such serialized architecture limits the frame rate to ~60 frames/s. In contrast, ultrafast ultrasound imaging utilizes the parallelization paradigm that emits plane waves at different angles and a contrast enhanced 2D image is obtained with coherent plane wave compounding[4]. The image frame rate is no longer limited by sequential scanning of the focused beam but by the travel time of the pulsed plane wave to propagate through the medium and get back to the transducer array, and the achievable frame rate is ~30 KHz for an imaging depth of 15 mm. Such high frame rate opens a wide range of possibilities for functional imaging technology development and will ultimately benefit human health care.
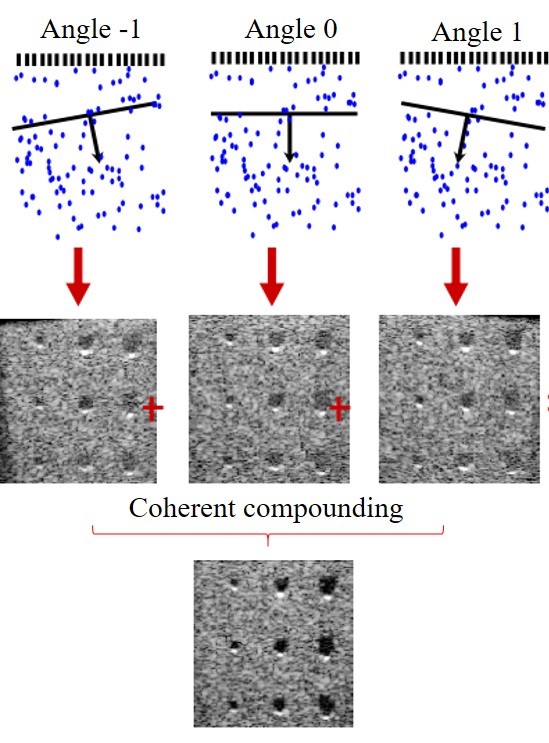
Fig 1. Schematic illustrating coherent plane wave compounding[4].
Power Doppler-based functional ultrasound imaging (fUS)
fUS was first introduced in 2011 by Mace et al [1] . It has the ability to image cerebral blood volume at ~100 micron spatial and sub-second temporal resolution with >15 mm penetration. In fUS, blood signal is obtained either by high pass filtering[1] or spatiotemporal clutter rejection[5], and the final image is formed by summing up the power of the blood signal for each pixel[1]. Fig. 2 shows three representative fUS images we have acquired at three different coronal planes.
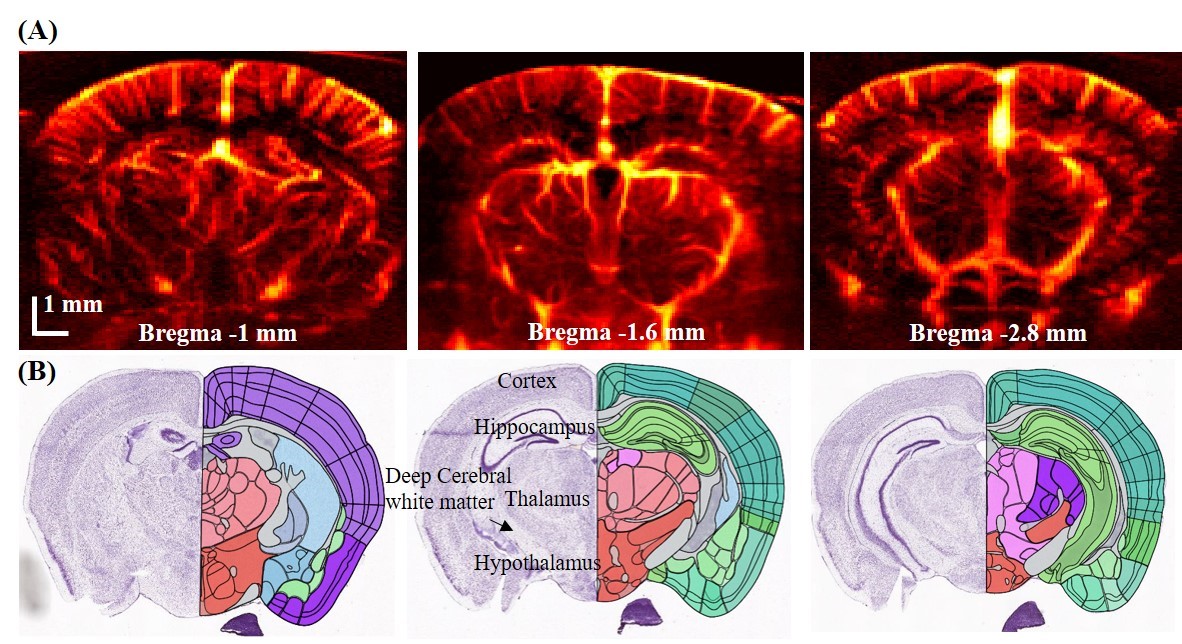 Fig 2. Power Doppler-based functional ultrasound image showing the entire coronal section of mice brain; (B) corresponding brain atlas.
Fig 2. Power Doppler-based functional ultrasound image showing the entire coronal section of mice brain; (B) corresponding brain atlas.
Microbubble-based ultrasound localization microscopy (ULM)
ULM[2] is an ultrasound analogue to optical super resolution technologies (e.g. photoactivated localization microscopy (PALM[6]), fluorescence photoactivation localization microscopy (FPALM[7]) and stochastic optical reconstruction microscopy (STORM[8])). It locates the centroid of flowing microbubbles and accumulates tens of thousands of such events to form a coronal plane blood vasculature with ~10 micron resolution. Fig.3 shows a representative ULM image we acquired.
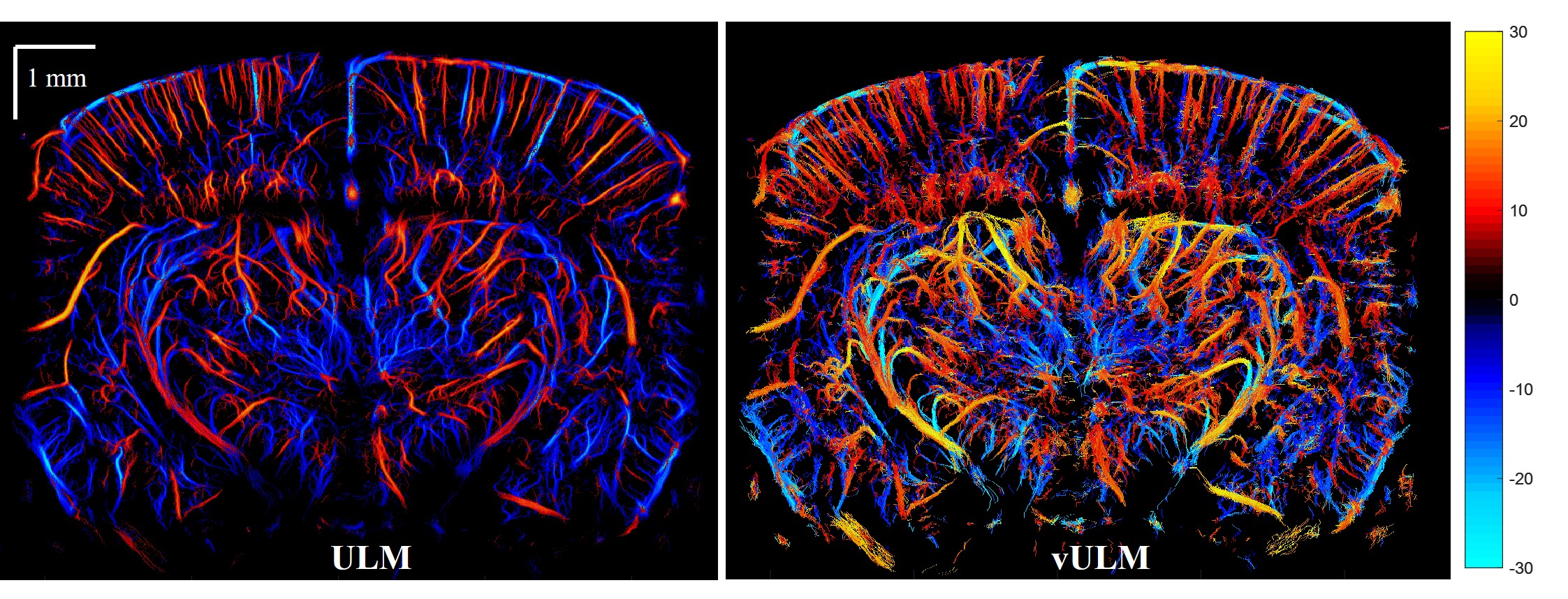
Fig 3. Ultrasound localization microscopy of a mouse brain.
Please contact David Boas if you need further assistance.
Reference
[1] E. MacÉ, G. Montaldo, I. Cohen, M. Baulac, M. Fink, and M. Tanter, “Functional ultrasound imaging of the brain,” Nat. Methods, vol. 8, no. 8, pp. 662–664, 2011.
[2] C. Errico, J. Pierre, S. Pezet, Y. Desailly, Z. Lenkei, O. Couture, and M. Tanter, “Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging,” Nature, vol. 527, no. 7579, pp. 499–502, 2015.
[3] M. Tanter and M. Fink, “Ultrafast imaging in biomedical ultrasound,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control, vol. 61, no. 1, pp. 102–119, 2014.
[4] G. Montaldo, M. Tanter, J. Bercoff, N. Benech, and M. Fink, “Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control, vol. 56, no. 3, pp. 489–506, 2009.
[5] C. Demené, T. Deffieux, M. Pernot, B. F. Osmanski, V. Biran, J. L. Gennisson, L. A. Sieu, A. Bergel, S. Franqui, J. M. Correas, I. Cohen, O. Baud, and M. Tanter, “Spatiotemporal Clutter Filtering of Ultrafast Ultrasound Data Highly Increases Doppler and fUltrasound Sensitivity,” IEEE Trans. Med. Imaging, vol. 34, no. 11, pp. 2271–2285, 2015.
[6] E. Betzig, G. H. Patterson, R. Sougrat, O. W. Lindwasser, S. Olenych, J. S. Bonifacino, M. W. Davidson, J. Lippincott-Schwartz, and H. F. Hess, “Imaging Intracellular Fluorescent Proteins at Nanometer Resolution,” Science (80-. )., vol. 313, no. 5793, p. 1642 LP-1645, Sep. 2006.
[7] S. T. Hess, T. P. K. Girirajan, and M. D. Mason, “Ultra-high resolution imaging by fluorescence photoactivation localization microscopy.,” Biophys. J., vol. 91, no. 11, pp. 4258–72, Dec. 2006.
[8] M. J. Rust, M. Bates, and X. Zhuang, “Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM),” Nat. Methods, vol. 3, no. 10, pp. 793–796, Oct. 2006.
[9] O. Couture, M. Ieee, V. Hingot, B. Heiles, and P. Muleki-seya, “Ultrasound localization microscopy and super- resolution : a state-of-the-art,” IEEE Trans. Ultrason. Ferroelectr. Freq. Control, vol. PP, no. c, p. 1, 2018.
