Laser Speckle Contrast Imaging
Real-time detection of blood flow changes with high spatial and temporal resolution can be a powerful tool for both experimental and clinical applications. Although Laser Doppler technique is a traditional approach for monitoring blood flow, it can only measure from a single point with very narrow (less than 0.1 mm3) field of acquisition. Over the last decade, Laser speckle contrast imaging (LSCI) is increasingly being used as a tool for quantifying relative changes in blood flow allowing preparation of two-dimensional maps for integration of spatiotemporal flow dynamics (Boas D & Dunn AK, 2010, J Biomed Opt, Dunn AK, 2001, JCBFM).
Principle
When a scattering surface is illuminated with laser light, due to random interference, a speckle pattern is generated. The speckle contrast over an area is expressed as the ratio of standard deviation to the mean speckle intensity in a window of pixels (usually 7×7 pixel grids).
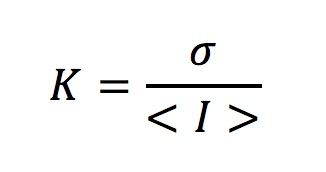
If there are moving scattering particles over the surface (like red blood cells) the speckle pattern fluctuates and speckles get blurred. This decreases the speckle contrast of the grid. Therefore, speckle contrast values yield information about the particle motion, i.e. blood flow velocity in a region of interest. Ideally, speckle contrast values are distributed between 0 and 1, where 1 represents no motion and 0 shows fastest motion to blur all the speckles. Speckle contrast is a function of the camera exposure time (T) and correlation time (time scale of the sample dynamics). Correlation time (tc) can be calculated with this information therefore, and it is inversely proportional to the speed of the scattering particles.
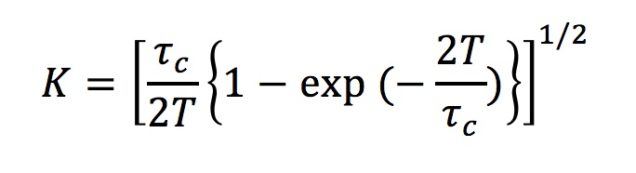
Ratios of correlation-time values acquired at different time points give the relative blood flow velocity changes at a certain time with respect to the baseline velocity.
Equipment
Laser speckle contrast imaging basically requires an illumination system, optical equipment and a CCD camera. A laser diode controlled by a laser power unit and a collimation kit are used to illuminate the surface. Visible red light or infrared range lasers can be used (700 to 920 nm). For optical acquisition, either a zoom lens connected to the camera or a microscope equipped with 4x-10x objectives can be used. Image acquisition is performed by a CCD camera. Raw speckle images can be acquired and processed online or offline with appropriate software environment to get two dimensional vasculature maps and for calculation of relative blood flow changes over time. The details for building a LSCI system can be found at Ponticorvo A & Dunn AK, 2010, J Vis Exp.
Here is a parts list for a system we built in 2018.

Applications
LSCI has been utilized extensively in the rodent brain, for blood flow during functional hyperemia (increased blood flow response in the cortex in response to sensory stimulation) (Dunn AK, 2003,Opt Lett) and neurological disease models of cortical spreading depression (Ayata C, 2004, JCBFM-1) and focal cerebral ischemia (Ayata C, 2004, JCBFM-2, Shin, 2007, Brain). It can be performed through a cranial window, thinned skull or even through intact skull in mice. Relative blood flow changes can be used for real-time guiding of the experiments and it can be easily combined with other imaging modalities like intrinsic optical signals, two-photon microscopy or optical coherence tomography (Yaseen MA, 2015, Biomed Opt Express).

Figure: Laser speckle imaging of the the mouse cerebral cortex through a cranial window. Left panel shows the baseline speckle contrast image of the cortical vasculature. Relative blood flow map showing the hypoperfused cortical area (right panel) during middle cerebral artery occlusion can be used to confirm and monitor ischemia during the experiment.
Laser speckle imaging has been utilized in some clinical applications as well, like monitoring of perfusion around skin lesions (Huang YC, 2008, Laser Surg Med) and also for intraoperative CBF monitoring to guide brain tumor resection surgeries (Richards LM, 2017, JCBFM, Richards LM, 2014, Neurophotonics).
GitHub codes:
Laser speckle processing code.
DLSI code.
Contact
Feel free to contact us (dboas@bu.edu) if you’re planning to implement this technique in your work.
