Mapping Molecular Pathways
 Swan, Goldberg and Ünlü: Shape Matters Swan, Goldberg and Ünlü: Shape Matters
DNA microarrays can monitor expression of thousands of genes simultaneously, making them powerful tools for biological research. These devices rely on hybridization, or the binding of sample DNA to oligonucleotide probes, short strands of DNA with a known sequence that are tethered to a glass slide. The pattern of where a sample has bound to the probes provides information about how genes are expressed in the DNA sample.

As powerful as microarrays are for researchers, they have not yet been approved by the Food and Drug Administration for diagnostic use. One reason is that the percentage of sample DNA strands that are able to hybridize fluctuates, making the process too imprecise for clinical purposes.
“To improve the hybridization efficiency of microarrays, researchers must understand the factors that influence how DNA binds to the array surface,” says physicist Bennett Goldberg. He is part of a team that has devised a new way to precisely measure the conformation, or shape, of DNA molecules tethered to a surface. “There are physical barriers that make it difficult to predict conformation,” explains Goldberg, “as well as differing densities of DNA on the array that complicate matters.” In addition, he says, a single strand of nucleic acids is floppy, like a soft rope, and can wiggle freely.
Goldberg’s collaboration with electrical engineers Anna Swan and Selim Ünlü, and former graduate student Lev Moiseev, has resulted in a new method called spectral self-interference fluorescence microscopy (SSFM). SSFM, Goldberg explains, is able to provide precise information about the size and shape of individual DNA molecules for the first time.
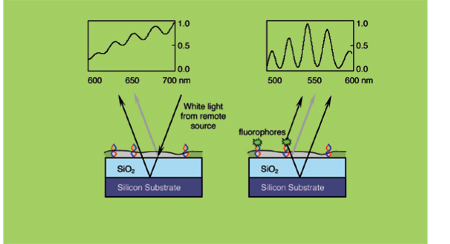
In SSFM, fluorescent labels are attached to one end of DNA tethered to a glass surface. Light is bounced off the top surface of the DNA layer, but it also travels through and bounces off a reflective bottom surface. The interaction of the two wavelengths—one reflected from the fluorescent label, the other from the bottom layer—produces oscillations in the spectrum of the returning light. The pattern of oscillations, or interference spectrum, describes the location and shape of the DNA molecule with sub-nanometer accuracy.
The team’s experiments showed that unhybridized single-stranded probes moved somewhat randomly, wriggling like worms at an average height of 2 to 2.5 nanometers above the surface. After hybridization, double-stranded DNA formed a rigid rod shape that was, on average, 5.5 to 10.5 nanometers from the surface and tilted at an angle of between 40 and 50 degrees relative to the surface.
Microarray manufacturers will soon be able to use SSFM to measure hybridization efficiency and optimize their product. According to Goldberg, SSFM can also be used to predict the virulence of viruses by measuring the conformation of sugars on their surface, and to study the shape of molecules on the surface of live cells. “One reason we care about DNA conformation is that it tells us about how molecules organize and act near surfaces,” he says. “Such information is crucial to design biosensors and biomimetic materials. It provides a template for measuring and controlling biomaterials at nanometer-length scales.”
This research was published in the February 2006 issue of the Proceedings of the National Academy of Sciences. For more information, visit http://nanoscience.bu.edu and http://ultra.bu.edu/projects.asp?project=fluorescence.
— by Leah Eisenstadt |






 Swan, Goldberg and Ünlü: Shape Matters
Swan, Goldberg and Ünlü: Shape Matters 
