The Race to a Battery-Powered Future
An automated electric car battery assembly line. A cleaner, greener energy future will require more efficient and more sustainable batteries—and BU researchers are already working on new alternatives. Video via iStock/SweetBunFactory
The Race to a Battery-Powered Future
Engineers at BU are figuring out how to make better, more sustainable batteries—a technology that is essential for clean energy
We know that to have a green future, the entire world needs to shift from fossil fuel–generated power to renewable energy. And as countries agree on tripling solar and wind capacity, there are still major hurdles in the plan: one is that existing batteries aren’t good enough.
The idea of storing energy for later use is old, but in order to move society toward clean energy, scientists and engineers are experimenting with the fundamental elements of batteries, finding better ways to source raw materials, and even testing more outlandish energy storage ideas—like electricity-conducting ceramics. Experts agree that batteries will be a vital resource to ensure power is always on tap, no matter when energy is collected from renewable sources—whether in very sunny months or in cloudy rainy seasons.
It’s been projected that the US will have over 26 million battery-powered electric vehicles on the road by 2030, most of which use lithium-ion batteries, the same kind as in laptops, phones, and other electronics. This will make the demand for battery minerals and metals higher than ever before. But is our current technology enough to power the future, and is it truly sustainable?
“If we look at really transitioning to electric vehicles, and to renewables that need more grid-level storage, we won’t be able to get there with just lithium ion,” says Emily Ryan, a Boston University College of Engineering associate professor of mechanical engineering. She studies alternative materials for constructing batteries. Mining current raw materials, like lithium and cobalt, can cause major environmental hazards and unsafe working conditions, and right now there’s no reliable way to recycle batteries once they’re spent, creating a waste nightmare.

“Batteries are a lot more complex than they seem, because they have all these impacts beyond where you’re using them,” says Benjamin Sovacool, director of BU’s Institute for Global Sustainability. “Truly sustainable solutions integrate mining, the design of batteries, all the way into waste.”
The future of batteries impacts us all—the materials they use, where the metals are sourced and mined, how they’re disposed of and reused. And all of the decisions and scientific discoveries made now will impact our future—like, how much greenhouse gas emissions will be averted by charging a car instead of filling the tank with gas? The race to better batteries is one that can change everything.
Reinventing Battery Architecture
Whether it’s in a phone, a plastic toy, or connected to a giant solar array, every battery’s purpose remains the same: store electricity until it’s ready to be used. And in all batteries, no matter the size or strength, there’s a delicate combination of chemistry and electrical engineering at play. Ryan and others at BU are figuring out how to improve the design of current batteries—for instance by swapping out the active layers of metal and adding different elements to make an old idea new again.
In her lab, Ryan uses complex computer models to test alternative battery materials, like lithium metal instead of lithium ion. According to Ryan, lithium-metal batteries, which use solid lithium metal as the anode (positive side), could have substantially higher energy density than lithium-ion batteries, which use a graphite anode. So, you can store more energy in the same size battery.
This video shows how lithium-ion batteries, which power everything from laptops to electric cars, charge and discharge. The cathode, on the left side, stores lithium and releases the ions when charging—so the ion particles move left to right. The anode, on the right side, releases ions when the battery is in use—so the ions move right to left. The separator in the middle keeps the negatively and positively charged electrons from touching. Video courtesy of US Department of Energy
“If we started using lithium-metal batteries in your cell phone, instead of charging it every day, you would charge it once a week. Or in a car with the same size battery as we have now, you might get 600 miles instead of 300 miles,” says Ryan, associate director of BU’s Institute for Global Sustainability. That also means a much smaller battery could be used to provide the same capacity as we have today (about a 300-mile range), so less materials would have to be sourced.
But lithium metal is far from perfect. It’s highly reactive and unstable, causing tree-like structures, called dendrites, to form as the battery goes through charging and discharging cycles. (All batteries get dendrites, but they’re more common in lithium metal.) Dendrites degrade the battery life and cause it to short-circuit. Ryan and her team, along with researchers at the Hebrew University of Jerusalem in Israel, are researching the root cause of dendrites by analyzing the interfaces of the material and the chemistry at play. Through a joint National Science Foundation-Israel Binational Science Foundation grant, they’re studying the chemical-physical processes occurring during battery operation with the aim of making things more stable. Ryan also tests other materials to figure out how to make batteries nonflammable, since fires are an all-too-common issue for e-scooters and other battery-powered devices.
“We know right now we are not on a sustainable path, so I think it’s on us to try and come up with solutions to help us get to a more sustainable energy generation and energy use,” Ryan says.
We know right now we are not on a sustainable path, so I think it is on us to try and come up with solutions to help us get to a more sustainable energy generation and energy use.
Dendrites and fires are only some of the consequences of design flaws in batteries. For a battery to store a lot of energy, it needs large electrodes—the anode and cathode on either end that the ions and electrons move between. But for a battery to charge quickly, it needs to be the opposite—the electrodes should be small layers, so the ions don’t have to travel as far from one side to the other.
“It’s really a conundrum in batteries, this idea of having as much energy as possible, but also being able to charge it quickly,” says Jörg Werner, an ENG assistant professor of mechanical engineering who studies what’s happening inside a battery cell, called the architecture of the battery. Werner has set out to solve that problem by focusing on how to make the layers of materials inside the battery as thin as possible, and interdigitated, like two interlacing hands. This shortens the distance between the negative and positive layers, allowing ions to move faster while making sure the positive and negative sides don’t touch, which would short circuit the battery.
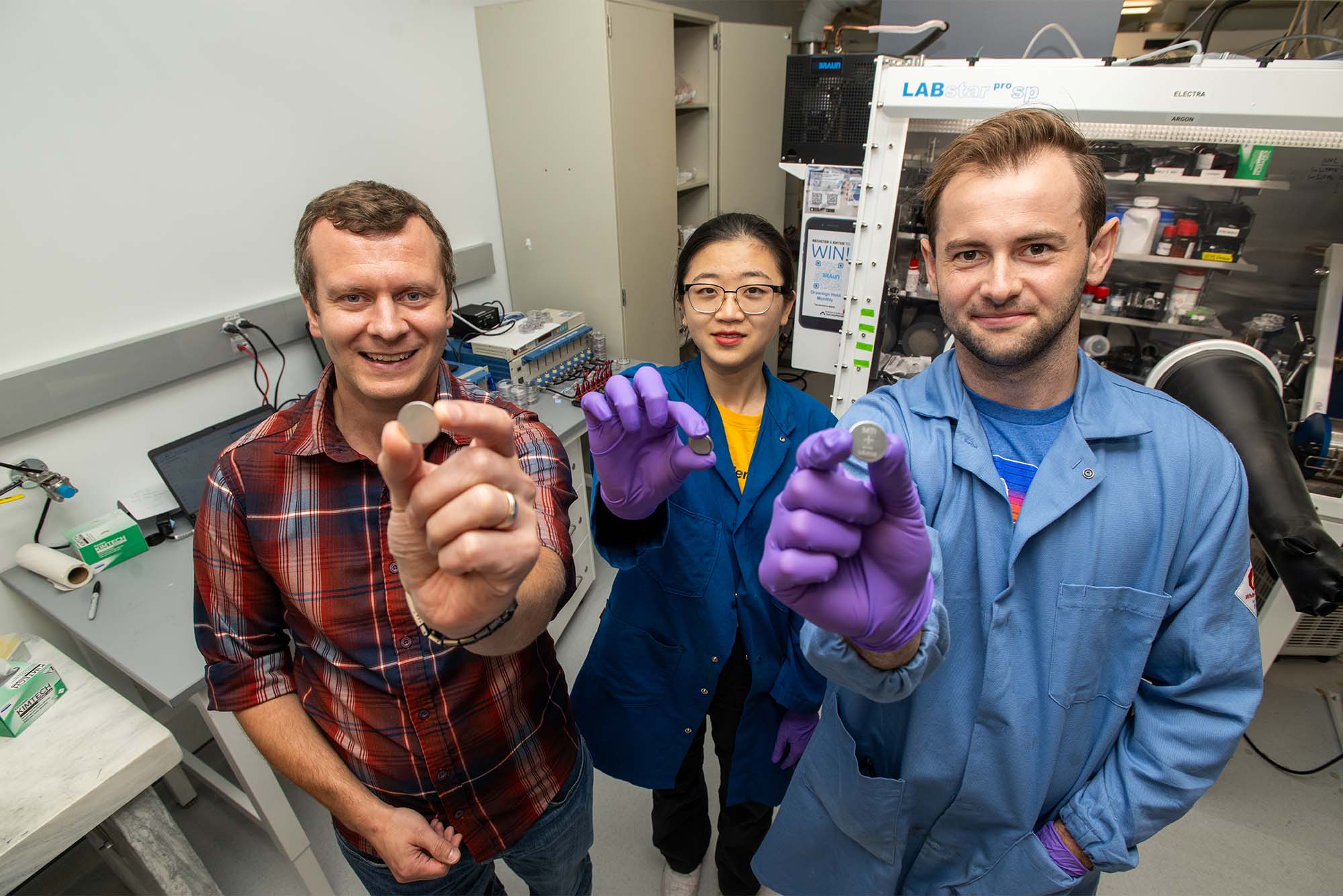
“That structure would allow us to have a very large fraction of active material, the material that stores all that energy within our battery cell, but at the same time we can have very short distances between the negative and positive active material to give us very fast charging,” he says. A design like this could help fuel the charge (no pun intended) of people adopting electric vehicles, since batteries would charge significantly quicker than current models.
The problem is actually making this new battery. Werner is working with Keith Brown, an ENG associate professor of mechanical engineering, to speed up the process of testing thousands of separator materials—the permeable membrane between the anode and cathode that prevents electronic short-circuits—using an autonomous system. They plan to test thousands of thin polymer separators, and Werner hopes to build a more advanced prototype in the near future.
“The architecture of the battery cell and all of its components have a huge impact on the performance of the battery that we as consumers actually care about,” Werner says.
Heat as Energy Storage
Large-scale battery storage capacity is expected to skyrocket over the next three years. And start-ups abound with long-shot battery solutions, like storing energy in cement to charge electric cars and converting iron to rust, and back again, as a method of storing and releasing energy.
“The bottleneck of going fully renewable is not a lack of technologies to harvest that energy,” says Sean Lubner, an ENG assistant professor of mechanical engineering. “One of the biggest technological barriers right now is energy storage.”
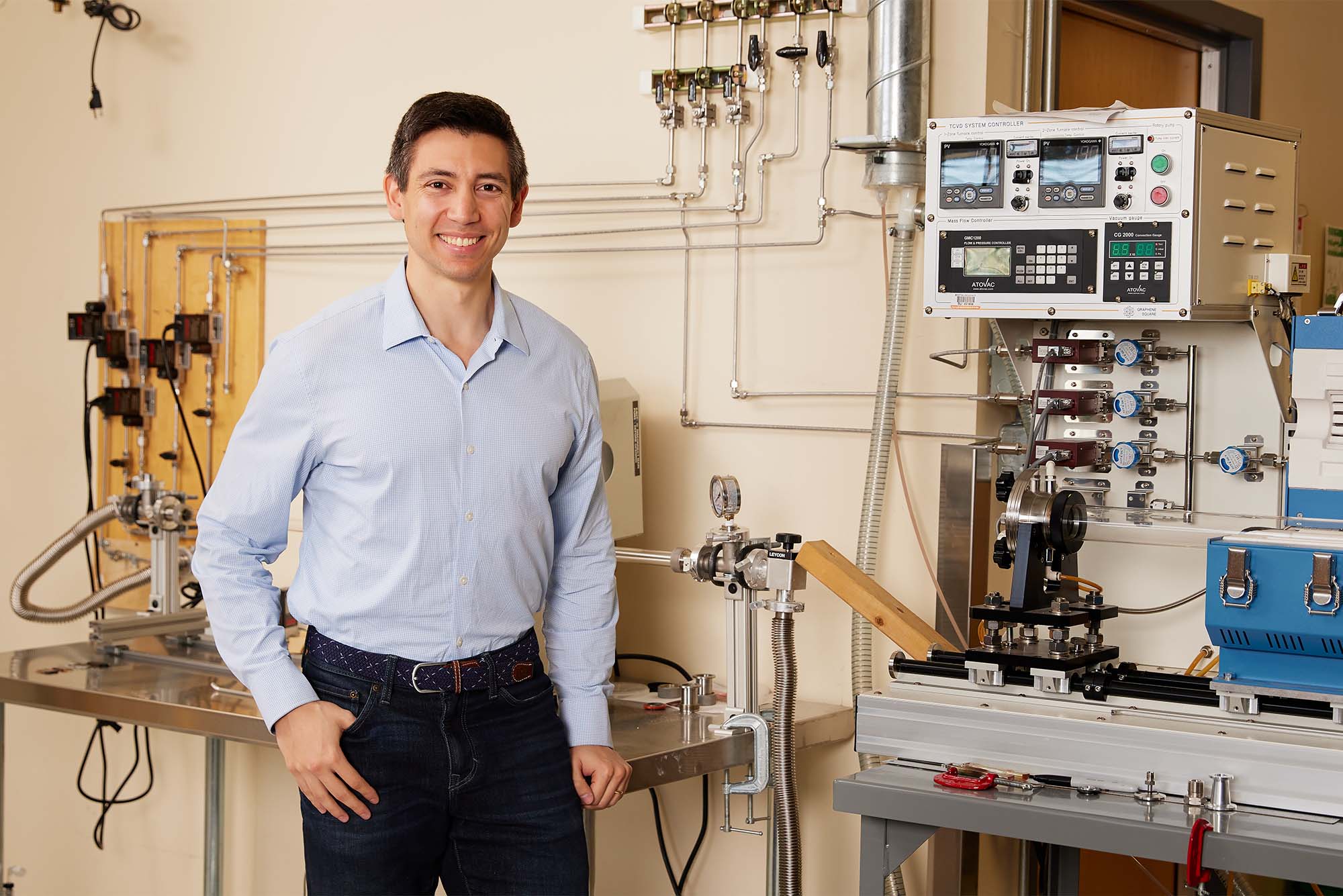
Imagine if the electricity powering your home was coming in from giant batteries charged with solar and wind energy, instead of from oil or gas, which account for the majority of the world’s greenhouse gas emissions. Lubner is researching how to use heat energy as a reliable and cheaper large-scale energy storage solution, as opposed to building expensive lithium-ion batteries. He’s developing an inexpensive, ceramic-based material that can safely store and conduct electricity even as it heats up to more than 1,200 degrees Celsius.
“The heat itself is the form of energy you’re using for storage,” he says. “Then later that energy can be converted back into electricity, or be released as heat.”
The idea is that electricity generated from wind and solar would be captured and converted into high-temperature heat. The heat can be stored for hundreds of hours until it’s needed, then used in different types of industrial processes or converted into electricity by a heat engine. In a recent paper, Lubner investigated the promising potential of thermal energy storage, describing how these systems would offer a cheaper alternative to lithium-ion batteries.
He and his team have shown that it’s possible to charge and discharge the material over 700 times, with the material able to withstand temperatures ranging from about 500 degrees Celsius to about 1,600 degrees Celsius. Even after such heavy use, “we saw negligible degradation to the properties, which is not typical of most materials,” Lubner says. “It will be necessary to make sure these materials have a long life, but I’d say it’s very reasonable to expect these to last 20 or 30 years with appropriate engineering.”
Reimagining Reusing
Even as these solutions swirl in laboratories, there’s the problem of how to better deal with the environmental impact of the batteries we have and need now—and will continue to need for many more years, until new options hit the market.
“Even 20 years ago, we were asking questions like, how do we build the stuff we need to fight climate change knowing that it’s still going to require energy-intensive materials like copper, steel, and concrete?” says Sovacool, who edited a book, The Routledge Handbook of Energy Security, which explores these very questions.
His research has pointed out that both ends of the battery life cycle—how battery materials are mined and sourced, and where the waste goes after the battery dies—pose massive environmental justice issues, safety hazards, and human rights risks.
“The process by which you get lithium at industrial scale can be as bad as mountaintop strip mining in West Virginia; we just don’t see it because it’s halfway around the world,” says Sovacool, also a CAS professor of Earth and environment. “I’ve met miners in the Congo who were digging mines without a shovel, literally digging the hole with their hands to get the cobalt or the copper.”
And then, at the other end, “we’re so bad at disposal,” he says. Less than 10 percent of battery waste is handled properly, meaning that most batteries end up in landfills, where they leak, explode, and pollute the environment. There are a number of searchable databases that locate nearby electronic waste recycling facilities, such as GreenCitizen, and mail-in services, but even more convenient options should be available, Sovacool says.
“Imagine if Energizer and Duracell gave you an envelope when you bought their batteries and, when they’re done, you mail it back to them,” he says. “These are extended producer responsibility schemes, where the battery companies say it’s on them to take it back and that creates a closed-loop supply chain.”
Imagine if Energizer and Duracell gave you an envelope when you bought their batteries and when they’re done, you mail it back to them.
The Global Battery Alliance has been working on this concept since it was founded in 2017, with the goal of creating a sustainable battery supply chain by 2030, including by safeguarding human rights and eliminating child labor. Last year, they launched a tool intended to increase transparency about whether car battery manufacturers are following sustainable practices, but it has limited information in its current state. According to Sovacool, the act of balancing truly sustainable practices with affordable batteries and electronics is where policy tools and experts stumble.
“The true tension is that you cannot have both cheap batteries and sustainable batteries,” Sovacool says. “If we start doubling the cost of batteries, what does that do to our low-carbon transition? What does that do to low-income families? Sustainability is important, but so is affordability.” For this, there’s no easy answer, he says, and remains a tension that urgently needs solutions implemented by global actors like the Global Battery Alliance.
With better design, industry standards, and affordable solutions, the future of batteries could be far different than we imagine now. And if we all want to do our part to prevent a climate disaster—with electric cars and solar-powered homes—it’s a future that can’t come soon enough.

Comments & Discussion
Boston University moderates comments to facilitate an informed, substantive, civil conversation. Abusive, profane, self-promotional, misleading, incoherent or off-topic comments will be rejected. Moderators are staffed during regular business hours (EST) and can only accept comments written in English. Statistics or facts must include a citation or a link to the citation.